Abstract
Introduction:
Several prognostic models for overall survival (OS) in chronic lymphocytic leukemia (CLL) have been developed, but none were derived in a relapsed/refractory (R/R) population or in the context of novel therapies. We therefore used a comprehensive model-building approach to derive and validate a novel risk model for OS in a population of previously treated patients (pts) with CLL who received therapy in randomized phase 3 trials of ibrutinib (IBR) vs standard of care (SOC) and idelalisib (IDELA) vs SOC.
Methods:
In collaboration with Janssen Pharmaceuticals, Gilead Sciences, and Pharmacyclics, the analysis included pts from 5 multicenter randomized phase 3 trials (n=1866): IBR plus bendamustine-rituximab (BR) vs placebo plus BR (HELIOS: NCT01611090), IBR vs ofatumumab (RESONATE: NCT01578707), IDELA plus BR vs placebo plus BR (Study 115: NCT01569295); IDELA plus rituximab vs placebo plus rituximab (Study 116: NCT01539512); and IDELA plus ofatumumab vs ofatumumab (Study 119: NCT01659021). All pts had a diagnosis of CLL, were previously treated, and required treatment.
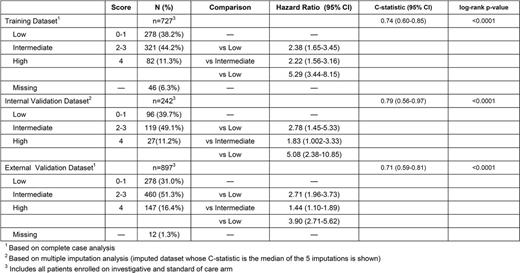
The model-building dataset(n=969) included pts from HELIOS and RESONATE, and was randomly assigned to the training dataset (n=727) and internal-validation dataset (n=242). The external-validation dataset was an independent pooled cohort from studies 115, 116, and 119 (n=897).
We applied univariate and multivariate analyses (MVA) to 28 candidate clinical, laboratory and molecular/genetic prognostic factors to derive the risk model in the training dataset. The primary endpoint was OS. We assessed the need for separate models for novel therapies by interactions with treatment in univariate analyses.
We evaluated different cutoffs for dichotomized covariates and for definition of risk groups after the final factors were selected. We fit a Cox regression on the training, internal-validation ,and external-validation datasets using the risk categories as the covariate to test the difference among the groups, and calculated the C-statistic as a measure of discrimination.
Results:
We proceeded with the development of a single risk score for all pts with R/R CLL, as only one potential prognostic factor (deletion 13q, interaction p value <0.10) demonstrated a differential effect necessitating separate models. Six factors were independently prognostic in the multivariate analysis of OS (p<0.05): β2-microglobubulin (B2M), lactose dehydrogenase (LDH), hemoglobin (HGB), immunoglobulin heavy chain variable region (IGHV) mutational status, number of prior therapies, and time from initiation of last therapy.
We excluded number of prior therapies from the final multivariate model, reasoning that its optimal cutoff might change with advances in CLL therapies. IGHV did not remain significant (p=0.0592) in MVA of 5 remaining prognostic factors. Notably, deletion 17p was not independently prognostic for OS.
The final risk model consisted of 4 prognostic factors: B2M ≥5 mg/L, LDH elevated, HGB <110 mg/L for women or <120 mg/L for men, and time from initiation of last therapy <24 months. Because hazard ratios of these factors were similar (range, 0.55-0.68) with overlapping confidence intervals, 1 point was assigned to each factor. The resulting score separated pts into low (score 0-1), intermediate (score 2-3), and high risk (score 4) groups.
Our risk model was prognostic for OS in the training dataset (log-rank p<0.0001; C-statistic 0.74 [95% CI 0.60-0.85]) and was confirmed in the internal-validation dataset (log-rank p<0.0001; C-statistic 0.79 [95% CI 0.56-0.97]), as well as in the external-validation dataset (log-rank p<0.0001; C-statistic 0.71 [95% CI 0.59-0.81]).
Conclusions:
We present the first validated risk model for OS in relapsed/refractory CLL in the era of targeted therapies. Our model consists of 4 readily available factors (B2M, LDH, HGB, time from initiation of last therapy) and identifies 3 prognostic groups with significantly different OS. This model reliably identifies high risk pts. Investigators can address this unmet need by designing prospective trials targeting these pts.
Darif: Janssen: Employment, Equity Ownership. Londhe: Janssen: Employment, Equity Ownership. Xing: Gilead: Employment, Equity Ownership. Huang: Gilead: Employment. Lin: Gilead: Employment. Byrd: Acerta Pharma: Research Funding; Genentech: Research Funding; The Ohio State University: Patents & Royalties: OSU-2S; Pharmacyclics: Research Funding; Janssen: Research Funding. Furman: Pharmacyclics: Consultancy, Honoraria; Genentech: Consultancy; Gilead: Consultancy; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy. Hallek: Abbvie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Hillmen: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Celgene: Research Funding. Jones: Sunesis: Other: Institutional research funding; Abbvie, Pharmacyclics, Genentech, Gilead, Janssen, Merck, and Acerta: Other: Institutional research funding; Genentech, Abbvie, Pharmacyclics, Gilead, Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sharman: Gilead Sciences, Inc.: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Genentech: Research Funding. Ferrante: Janssen: Employment, Equity Ownership. Reddy: Pharmacyclics/Abbvie: Employment, Equity Ownership. Dreiling: Gilead Sciences, Inc.: Employment. Adewoye: Gilead: Employment. Dubowy: Gilead: Employment. Howes: Janssen R&D: Employment, Membership on an entity's Board of Directors or advisory committees; Johnson & Johnson: Equity Ownership. James: Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Zelenetz: Celgene: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal